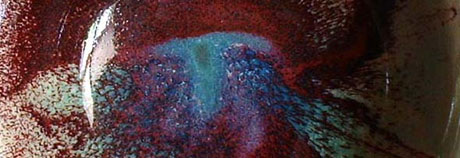
Copper reds, of all the classic high-fired reduction glazes, are the most fascinating and the most frustrating. Just when certain effects seem under control, they change completely or even cease to happen at all. For a while I tried to achieve consistent results, but eventually I realized it was not feasible or even desirable, and began to enjoy the wild variations.
Copper reds date from the Chinese Tang Dynasty (618-907) and became a major part of the glaze repertoire of the Sung Dynasty (960-1279). Advances in kiln technology had seen the development of high-temperature kilns, making it possible to fire stoneware and porcelain clays with simple glazes composed mainly of feldspar. The wood-burning kilns had a naturally *reducing atmosphere which produced the beautiful green and blue celadons from iron and red from copper.
*(Containing free carbon due to unburned fuel; the carbon "steals" oxygen from the metallic oxides in the glaze and body which forces them to change in molecular composition, reflecting light differently and thus changing the colour).
What actually happens chemically is this: When fired in the kiln with an ample supply of oxygen, the copper oxide molecule in the glaze is in the form of cupric oxide (CuO) which is usually green or black. However when fired under reducing conditions, the carbon (C) molecules in the oxygen-starved atmosphere draw oxygen from the cupric oxide, which is the least stable component of the glaze, to form carbon dioxide (CO2), leaving cuprous oxide (Cu2O) and/or colloidal copper (Cu) (incredibly small--4/100,000ths of an inch diameter--metallic copper particles). The colloidal copper is what produces the beautiful rich red in a glaze.
Copper carbonate(CuCO3) is a good form of copper to use in a glaze, because it contains a carbon atom already: CuCO3 + heat = CuO (Cupric Oxide--vulnerable to attack from free carbon) + CO2.The more I learn about these glazes the more I am convinced that they are affected as much by the firing as by the glaze composition. Peach bloom, sang de beouf, rouge flambe can all happen with the same glaze on the same clay, fired in different kiln loads. And they can be completely different from one place to another in the kiln. After firing an entire load of copper-glazed pots, I found that my last kiln produced the best reds in the bottom front right hand corner, right by the burner; but in my present kiln, virtually the same as the old kiln, reds turn out best in the centre.
Almost any clear base glaze will work with copper. For the clearest reds, the glaze should contain very little clay. Clay in the glaze makes it stiffer, thus less likely to run, so copper reds are notoriously runny. Only a tiny amount of copper is necessary to achieve a good red, usually about .3 per cent by weight. Adding about triple that amount of tin will help develop the colour. The glaze coat should be quite thick or it will be colourless.
I've found that chrome green can interfere with the development of copper reds, so I avoid putting pots with a lot of chrome green in the same firing load, but chrome green leaves painted on pots along with red and purple flowers don't seem to hinder it.
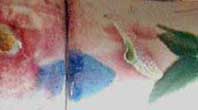
I've found too that a pot glazed with Bobby's Blue Glaze (recipe follows) placed in the middle of a group of copper red glazed pots usually enhances their red remarkably. Evidently the excess copper migrates to the other pots. The barium-glazed pots turn out radically different every time, with all sorts of interesting patterns. These are some examples:

And I've found that the best way to get spectacular copper reds is to not expect them, (and to fire lots of copper-glazed pots).
Note: there is an excellent article in the September 2021 issue of Ceramics Monthly magazine "Copper Red Nanoparticles" by Karthik Lalwani, Michael Leopoldm PhD, and Ryan Coppage PhD.